Abstract
Introduction: Pain, anxiety, depression, and other aspects of health-related quality of life are important issues for people with hemophilia and for caregivers of children with hemophilia. These aspects may be measured using patient-reported outcome (PRO) instruments; however, the use of these instruments in clinical management of patients with hemophilia is limited and inconsistent. This analysis of data from the B-HERO-S study aimed to assess correlations between PRO domain scores and psychosocial questions commonly asked in comprehensive care settings.
Methods: The B-HERO-S study consisted of 2 online surveys, one administered to adults (aged ≥18 years) with hemophilia B (n=299) and one administered to caregivers of children (aged <18 years) with hemophilia B (n=150). Each survey included PRO instruments (patients: EQ-5D-5L with visual analog scale, Brief Pain Inventory v2 Short Form [BPI], International Physical Activity Questionnaire, Hemophilia Activities List, and Patient Health Questionnaire [PHQ-9]; caregivers: PHQ-9 and Generalized Anxiety Disorder [GAD-7]) and questions related to demographics, hemophilia treatment, and other characteristics. This post hoc analysis assessed correlations between responses to selected questions with BPI pain severity/interference, PHQ-9, and GAD-7 scores via Pearson correlations. Correlations ≥0.37 were considered high.
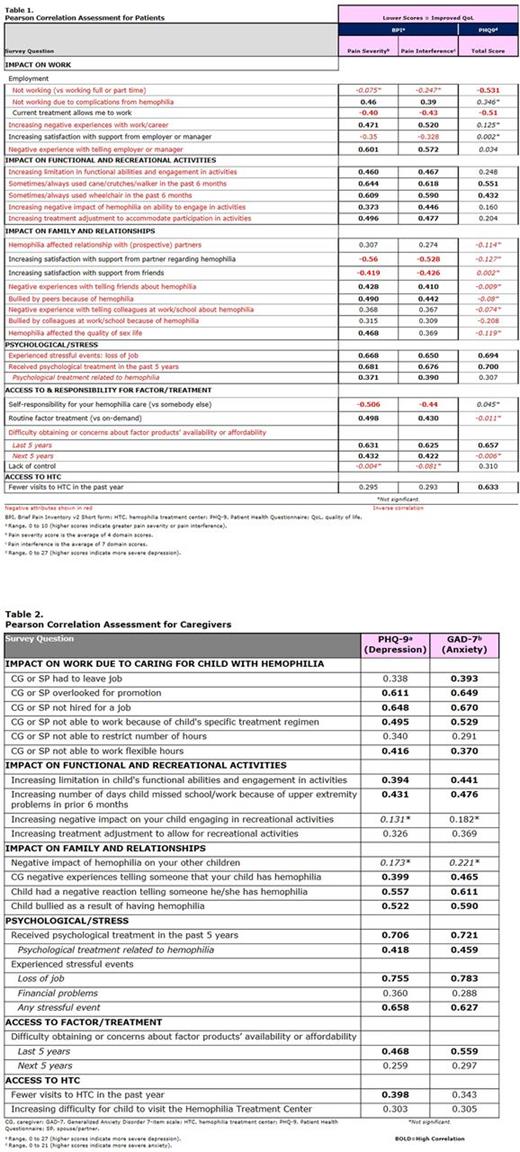
Results: For adults with hemophilia B, common questions significantly correlated with BPI pain severity/interference were highly similar and of expected directionality (Table 1). Greater pain severity and pain interference were each associated with employment issues (including not working due to hemophilia complications and negative experiences with work), functional limitations (including use of cane/crutches/walker or wheelchair in the past 6 months and negative impact of hemophilia on engagement in activities), relationship issues (including low satisfaction with support from partners or friends and being bullied by peers because of hemophilia), psychological issues (including stressful events and having received psychological treatment), and treatment issues (including low self-responsibility for care and having/expecting difficulty accessing factor due to availability or affordability). In comparison, fewer responses were significantly correlated with higher PHQ-9 depression scores; these included working, not being able to work on current treatment regimen, using a cane/crutches/walker or wheelchair in the past 6 months, experiencing a stressful event, receiving psychological treatment, having had difficulty accessing factor/treatment, and having less frequent HTC visits.
For caregivers, questions significantly correlated with PHQ-9 (depression) and GAD-7 (anxiety) scores were similar and of expected directionality (Table 2). Greater depression and anxiety were each associated with employment issues (including caregiver or spouse/partner having not been hired/promoted or not being able to work because of their child's treatment requirements), their child's functional issues (functional limitations and having to miss school/work because of upper extremity problems), relationship issues (including negative experiences telling others about their child's hemophilia or their child being bullied because of their hemophilia), psychological issues (including having received psychological treatment and experienced stressful events), having had difficulty or concerns with treatment/factor availability or affordability, and having less frequent HTC visits.
Conclusions: These data demonstrate high correlations (≥0.37) between PRO instruments measuring pain, depression, and anxiety and many questions that may be asked of patients and caregivers in the comprehensive care setting. In adult patients, more questions were significantly associated with pain severity/interference than with depression. In both adult patients and caregivers, responses significantly associated with depression/anxiety seemed to reflect acutely impactful issues (eg, recent problems related to functional impairment or disability, recent difficulty or concerns with factor availability) vs more historical issues (eg, negative experiences in telling others about their or their child's hemophilia, being bullied or their child being bullied because of hemophilia).
Buckner: Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Uniqure: Consultancy; Shire: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees. Sidonio: CSL Behring: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Aptevo: Membership on an entity's Board of Directors or advisory committees; Biogen: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Witkop: Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen Idec: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptevo: Membership on an entity's Board of Directors or advisory committees; Baxter Bioscience: Membership on an entity's Board of Directors or advisory committees. Guelcher: Solution Sight: Speakers Bureau; Octapharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Grifols: Membership on an entity's Board of Directors or advisory committees; Baxter/Baxalta: Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Membership on an entity's Board of Directors or advisory committees. Cutter: Pfizer: Membership on an entity's Board of Directors or advisory committees; Biogen: Honoraria. Iyer: Novo Nordisk Inc.: Employment. Cooper: Novo Nordisk Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal